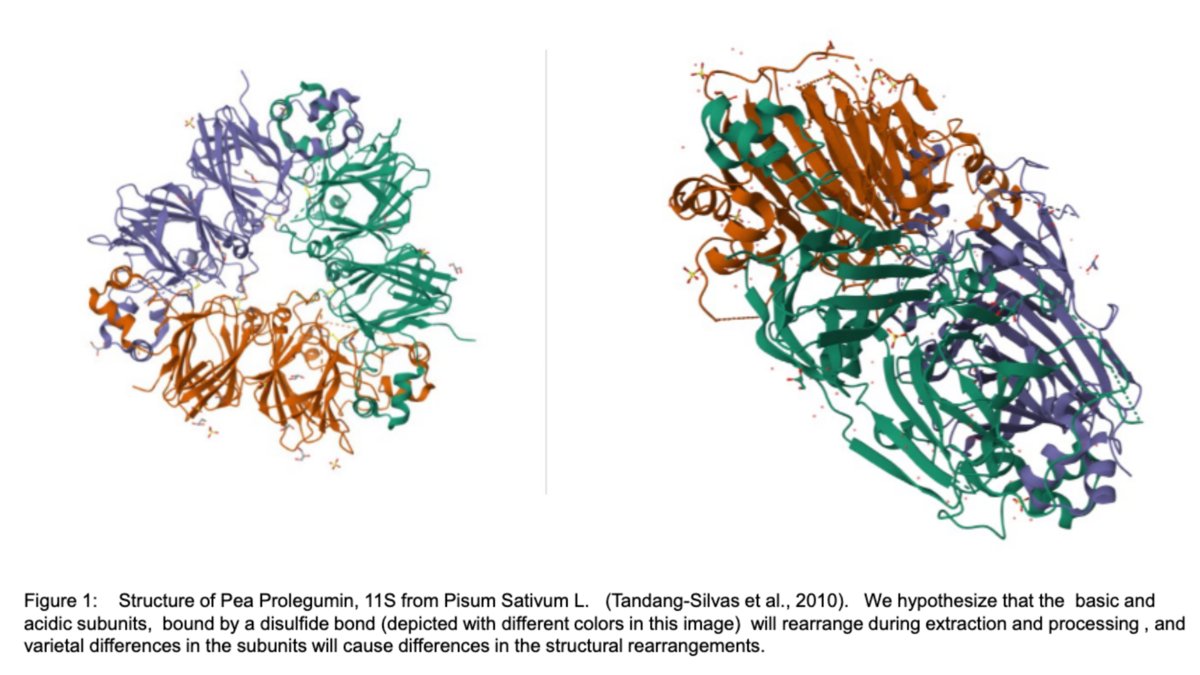
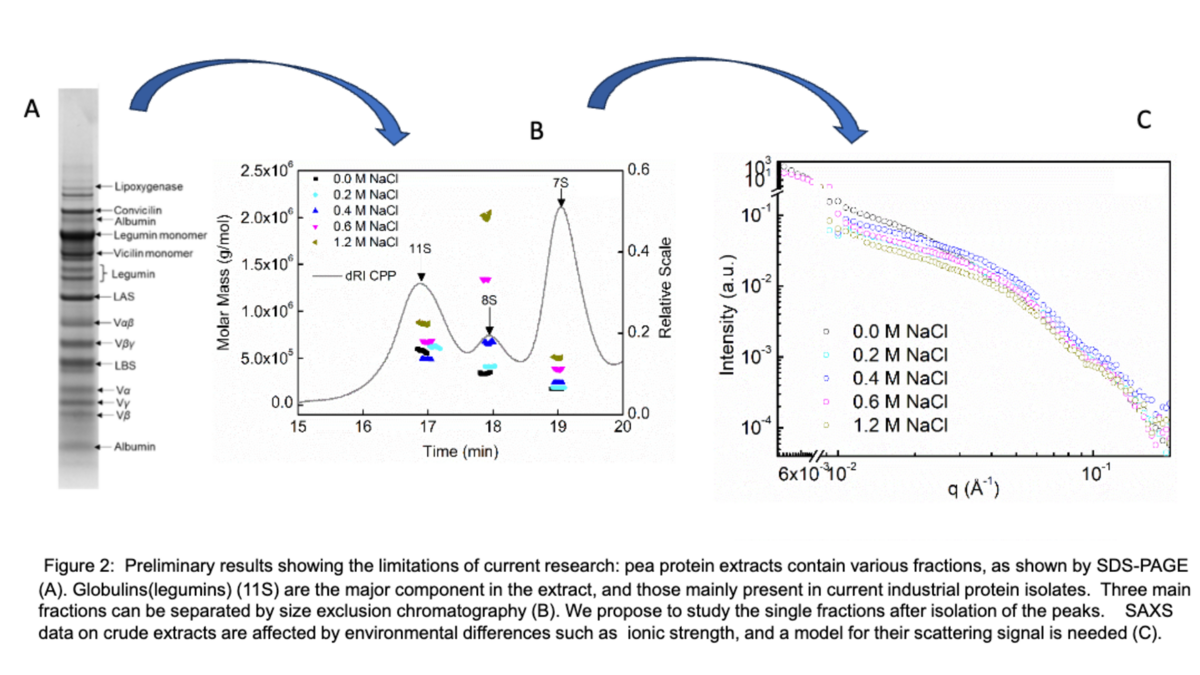
SupraPEA
"Composition and processing factors affecting the supramolecular structure of pea globulins"
The SupraPEA project aims to bridge the current knowledge gap related to the interplay between composition, environmental factors, and processing conditions. This interplay determines the structural changes which ultimately affect the technological properties of pea protein ingredients.
⌐ The project has been granted 2.956.863 DKK in funding.
⌐ The project starts on 1 June 2024 and will end on 31 May 2026.
Introducing the SupraPEA project
To better utilize plant globulins, we need to understand their structural changes at various length scales
The SupraPEA project aims to bridge the current knowledge gap related to the interplay between composition, environmental factors, and processing conditions. This interplay determines the structural changes which ultimately affect the technological properties of pea protein ingredients.
Background for the project
Current utilization of plant protein ingredients is hampered by the inability to predict their properties during processing. Isolates are obtained by various aqueous extractions, concentrations, precipitations and drying. Not only the composition is affected, but more importantly the structural changes induced during these processes. It is therefore important to provide new fundamental knowledge on the conditions, compositional and genetic variability and processing factors that when applied during the isolation affect their structural rearrangements and aggregation, leading to critical differences in the technological functionalities.
The activities
The SupraPEA project will evaluate how subunit compositions, lipids components, ions and other environmental factors affect pea protein structure and function. A model for composite, polydisperse globulin particles, will be developed and linked to fundamental functionality studies, using rheometry, tensiometry. Together with industrial partners, the project's academic researchers will then utilize this structural model at various length scales to evaluate pure globulins, laboratory and commercial isolates and derive some general principles towards their production and utilization
The outcome
This research will empower the entire scientific community with a more basic understanding of the structure of pea proteins at various length scales, and how modifications of such structures during aggregation and their polydisperse characteristics determines changes in functionality. The results will provide breeders new knowledge to select for varieties with best functionality, academic researchers with robust models for data interpretation, and manufacturers and end users with tools for tailoring the functionality of the final ingredients and minimize processing.