Developing a protocol for growing microscopic human brains in the lab
The MiCO Platform team wanted to develop a model for better mimicking of human neurological disorders. Read here, how the team developed a protocol for growing microscopic brains from human stem cells.
"We are eager to share the MiCO Platform with as many labs as possible. It’s a great advantage that other researchers can give us feedback and suggest modifications, which we can implement into the system to make it even more representative for neurological disorders,” says Mark Denham, who was heading the MiCO Platform project, and continues: “Openness and collaboration are key to finding great solutions and making them accessible to everyone. ODIN has provided an ideal setting for this.”
During the last three years, the MiCO Platform team has developed a protocol for generating microscopic brain models. Therefore, the MiCO Platform now provides an alternative to both traditional cell cultures and the use of animal models when screening drug candidates for neurological disorders before clinical trials.
From mini to micro – the MiCO Platform team generated micro brains
As is the case for many other disease areas, drugs developed for neurological disorders often fail in clinical trials. “There are basically three general model systems: 2D cell cultures, human organoids, and animal models. Each have their pros and cons, but the bottom line is that none of them fully represent the complexity of the human brain or do it in a reproducible way,” Mark Denham says and thus pinpoints the most important aspect of a good model system for the human brain.
He explains that the 2D human stem cell cultures struggle to survive over a longer period of time, and that the cultures lack the supporting cell types to properly mimic the in vivo environment. In contrast to this, the animals are in vivo models with complex brain structures where the different types of neurons and a plethora of supporting cells are intact. “Even when you use animal models, it is not possible to mimic the human central nervous system. That’s why researchers developed organoids – or mini brains – that provide a more complex model,” explains Mark Denham. “You can grow these mini brains in the lab in plastic wells from human stem cells. When the stem cells divide, they develop into brain-like structures representing several cell types. The mini brains are quite complex and have several advantages for disease modelling and understanding. But they also come with some limitations.”
Although the nickname for organoids is mini brains, their limitations primarily arise due to their relatively big size (1-1,5mm): First, the cells in their centre often die, because of an inefficient nutrient flow. Second, they are rather disorganised, which makes it difficult to reproduce results. Lastly, the organoids are too big for high-throughput screening of drug candidates. “In the MiCO Platform team we wanted to address exactly these issues,” Mark Denham says. “We decided to develop a protocol for generating miniaturised controlled organoids – MiCOs or micro brains – that represent different parts of the brain. When the micro brains only mimic either the forebrain, the midbrain, or the hindbrain, fewer cells are needed to start with. The mature micro brains are only about 0,5 mm wide, which means that the centre cells don’t die. Also, the micro brains are faster to generate, easier to reproduce, and they can be used in large scale screening protocols.”

The generation of micro brains requires reprogramming of cells
In the lab, the MiCO Platform team can generate micro brain from basically anyone as long as they have a cell sample from them. “We can take a patient’s cells and reprogram them back to stem cells – the so-called induced pluripotent stem cells,” explains Mark Denham. This new stem cell line is then programmed a new. The MiCO Platform team can force the stem cells to develop into micro brains with neurons that represent this specific patient’s neurological disease.
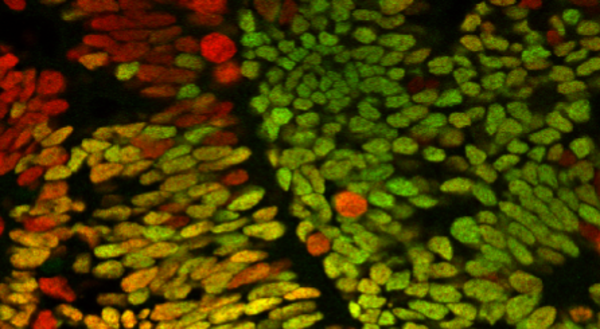
Based on the protocol used, the reprogrammed cells can form the basis for different areas of the brain. Micro-forebrains are specifically well-suited for studying Alzheimer’s disease, whereas the micro-midbrains are optimal if you are interested in Parkinson’s disease. Studies in ALS and spinal cord injury can be performed in the micro-hindbrains. To ensure that the micro brains mimic their human counterparts, the MiCO Platform team was keen on also including the supporting cells known as glial cells. The presence of glial cells enhances the maturation of the neuronal cells, it keeps the micro brain healthy, and it helps mimic in vivo cellular diversity. “We can grow the micro brains for at least 150 days in the lab,” Mark Denham says and elaborates: “When we generate the micro-midbrains, we already have a nice population of postmitotic neurons at day 75. Later, we see some glial cells developing from the progenitor cells. This process pretty much mimics what happens during development of the human brain.” The MiCO platform team is excited about the presence of glial cells, because the interplay between neurons and glial cells are often important in neurodegenerative disorders.
Drug candidate screening and cell therapies – the potential of micro brains
The aim of the MiCO Platform project was for the micro brains to be used in e.g., large-scale screening of drug candidates for neurodegenerative disorders. Such a human in vitro model will help pharma companies to reduce both the amount of time, the costs, and the number of animals used in research and development. As a first test in drug candidate screening, the MiCO Platform team generated micro brains from both a patient with Parkinson’s disease and a healthy control and subjected them to potential drugs targeting Parkinson’s disease. “The micro brains grown from diseased cells were used to test efficacy towards Parkinson’s diseases, whereas the healthy micro brains served as a control for toxicity,” says Mark Denham. As a platform control the team administered a known drug to verify that the expected response was observed in the cells.
Several companies are expected to be interested in a platform like the MiCO Platform. Both as drug screening platform but also as a platform for testing cell transplantations, where new healthy cells replace diseased cells within the patient. In neurodegenerative disorders, the idea is to transplant healthy cells into the patient’s brain and therefore the micro brains can potentially replace the use of animal models. To identify the potential of the micro brains as an alternative to animal models, the MiCO Platform team generated cells for transplantation and transplanted them into rats and grew them as micro brains. “We expected the transplanted cells to acquire a mature phenotype in both model systems,” Mark Denham explains. “Our single-cell RNA sequencing results show that there are indeed similarities between the cells matured in both model systems although there are also some differences in maturation rate."
The Future of the MiCO Platform
Despite the differences in the results obtained in animals and the MiCO Platform, Mark Denham is confident that the MiCO Platform has potential to become an important platform in the future: “The MiCO platform presents a valid model for applications like drug screening potency assessments, and co-culture optimization. Overall, our results from the MiCO Platform project demonstrate the utility of the MiCO system for drug screening or as in vitro potency assays for cell therapies."
Although the project has officially ended, the MiCO Platform team welcomes input from other researchers. Their goal is to develop the platform even further. So far, the team has published the protocol for generating the micro-midbrains and the protocol is already used by several groups at Aarhus University. “We have received a lot of interest from other research labs, and we are happy to share our knowledge and protocols with others,” says Mark Denham. In the future, the team aims for both new academic and industrial collaborations. “We hope to generate even more complex micro brains – for instance with co-culturing of other types of glial cells. We are also interested in developing new protocols with other cell types,” Mark Denham concludes.