Yonehara group at DANDRITE have identified a new circuit mechanism in mammalian retinal motion computation
Latest research from the group led by Keisuke Yonehara at DANDRITE has recently been published in peer-reviewed scientific journal “Current Biology”. The study is about the space-time wiring between a type of motion-sensitive cells that project to the brain for gaze stabilization and local excitatory cells in the mouse retina.
Akihiro Matsumoto is a postdoctoral fellow in Yonehara’s group with a strong interest in the circuit basis for visual processing in the retina. Here, Matsumoto talks about their findings.
The visual system is a remarkable part of the central nervous system. Can you elaborate on your main focus in the visual system and introduce your project that led to your latest publication?
In the mammalian nervous system, the retina is the first stage of visual motion processing. Retinal direction-selective (DS) cells have a tuning in response to one specific motion direction. The tuned responsive direction is called "preferred direction", and the opposite, unresponsive direction is called "null direction". The previous studies showed that inhibitory inputs in null direction establish DS responses, however, the contribution of "excitatory inputs" is unknown. In addition, there are two major subtypes of DS cells; there are the ON-OFF DS cells and ON DS cells. The ON DS cells have clear speed sensitivity in the range of slow-speed but, the mechanisms underlying the speed-sensitivity is unknown. Since we do not know why excitatory inputs become direction-selective, the main purposes with our study, are to examine the contribution of excitatory inputs to ON DS cells and the mechanisms for their slow-speed sensitivity.
Can you tell me about your main findings?
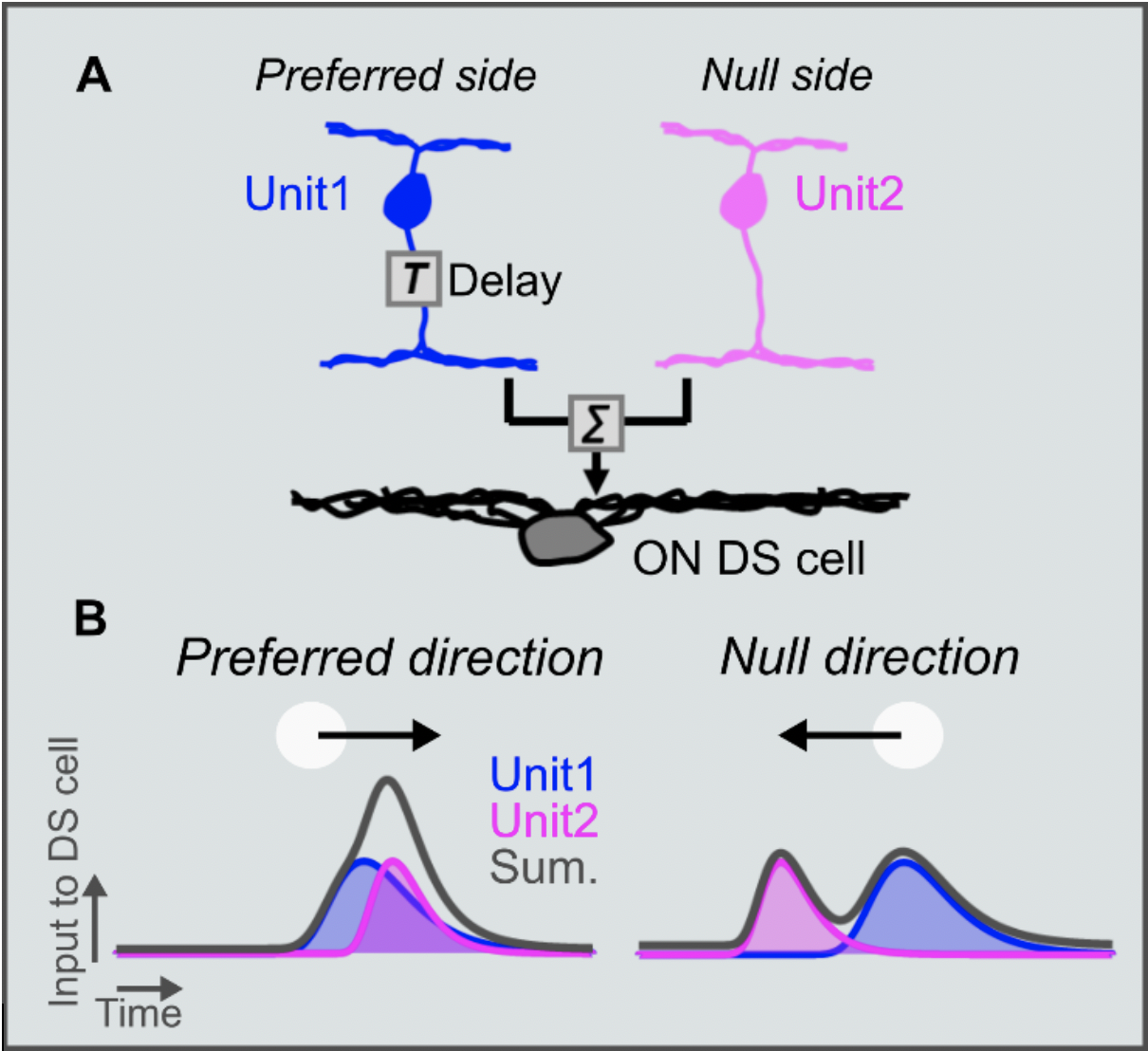
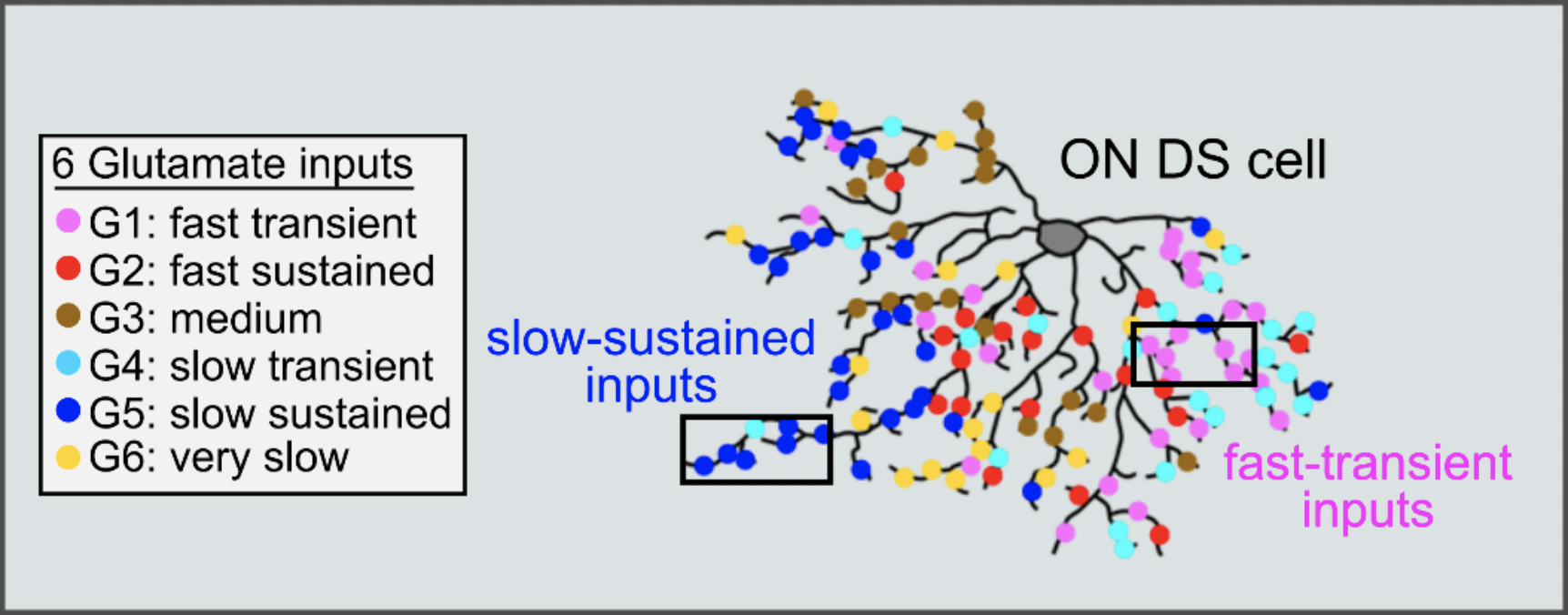
We hypothesized the mechanism to explain direction selectivity in excitatory inputs and created a model in which we predicted two different units (unit1 and unit2) with distinct delays. The earlier activation of unit1 is delayed, and then is effectively integrated with the later activation of unit2 resulting in the enhancement of excitatory inputs in the preferred direction. The excitatory inputs are mediated by glutamate release from bipolar cells and we therefore preformed two-photon glutamate imaging to monitor the glutamate inputs. Using statistical clustering methods, we characterized the glutamate inputs based on the temporal dynamics and discovered "6 subgroups" with different temporal dynamics. We were able to find "fast units” and "slow units", that supported our model. We also needed to spatially organize the fast and slow units and therefore mapped the location of the 6 groups of glutamate inputs. The spatial organization that showed that the slow units were located at preferred side, and the fast units were located at null side, was supported by an anatomical study using EM reconstruction, performed by Kevin L Briggman (CAESAR). Finally, we established a simulation model to examine the impact on direction selectivity and speed sensitivity. We found that the offset of synaptic delay to enhance excitatory inputs works in the range of slow-speed. To conclude, ON DS cells have a circuit mechanism to amplify excitatory inputs in the preferred direction by the offset of synaptic delay. The synaptic delay across the inputs are offset in slow-speed effectively, resulting in the slow-speed sensitivity in ON DS cells.
What has been the most challenging aspect in this project?
Matsumoto comments; “From the technical point-of-view the challenging aspect was to establish and optimize the systems and analysis we use, and we needed to systematically and statistically define or characterize for certain what is a faster input and what is a slower input. Kevin L. Briggman (CAESAR) is an expert in electron microscopy and was able to map the subtype of bipolar cells anatomically and was able to find the asymmetric wiring of bipolar cells and DS cells. Another challenging aspect was the difficulty to validate our model for the contribution of excitatory inputs to the direction selectivity. It has been a kind of "dogma" that inhibitory inputs have significant contribution to direction selectivity, and the contribution of excitatory input is little. So, to update the dogma, we needed to validate our results based on the different methodologies: electrophysiology, two-photon glutamate imaging, anatomical study, and model simulation. The correction of these evidences from different approaches gave novel functional insight into excitatory pathway: speed sensitivity”.
Can you tell a little about what you will be looking into next in terms of this paper?
Matsumoto elaborates; “At the moment we are studying the mechanism of the ON-OFF faster tuning speed DS cell. Also, in this paper, we study the glutamate from the bipolar cell, but glutamate is not the only excitatory synaptic transmission for the retina. Using the same approach as in this paper following modification of the applied model, we are also interested in studying acetylcholine transmission”. This excitatory mechanism would be potentially interesting for clinical application. Idiopathic congenital nystagmus is a severe eye-movement disease in humans which affects roughly 1 in 1500 people, and is caused by a mutation in the FRMD7 gene. The study developed the mouse model showed that the mutation of Frmd7 gene affected specifically inhibitory DS mechanism (Yonehara et al. 2016, Neuron). That means, the excitatory DS mechanism still remains in the patients. Now we are trying to develop the genetic strategies based on the excitatory DS mechanism for restoring retinal computation.
To read the publication, “Spatiotemporally Asymmetric Excitation Supports Mammalian Retinal Motion Sensitivity” in full, visit the Current Biology website.
Visit Current Biology to read the dispatch article on Yonehera’s latest publication.
For more information on the Yonehara group and their research, visit the DANDRITE website.
Also visit Yonehara Lab for additional insights on publications, people and lab life.