Molecular profiling to reveal how memories are stored
Meet DANDRITE Group Leader, Taro Kitazawa, and learn how he is looking for epigenetic and transcriptional mechanisms that underlie neuroplasticity.


When a child learns how to ride a bicycle, a memory of that new experience is formed. It is encoded in the child’s brain circuits and stored. This learning and memory formation from new or sudden experiences requires plasticity, or the ability of the brain to change its functional structure or circuitry. Genomic and epigenomic molecular events underlie such plasticity.
Dr. Taro Kitazawa recently joined the Danish Research Institute for Translational Neuroscience (DANDRITE) to look at the molecular basis of this neuroplasticity. In particular, he is trying to discover the key epigenetic and transcriptional events that occur when the brain undergoes the drastic changes of new memory formation.
Dr. Kitazawa explains, “when we experience something, signals are transduced to our brain. Then our neurons are activated, making action potentials that cause sequential induction of different types of genes.”
Just how many genetic and epigenetic changes could be taking place when new memories are formed? He explains:
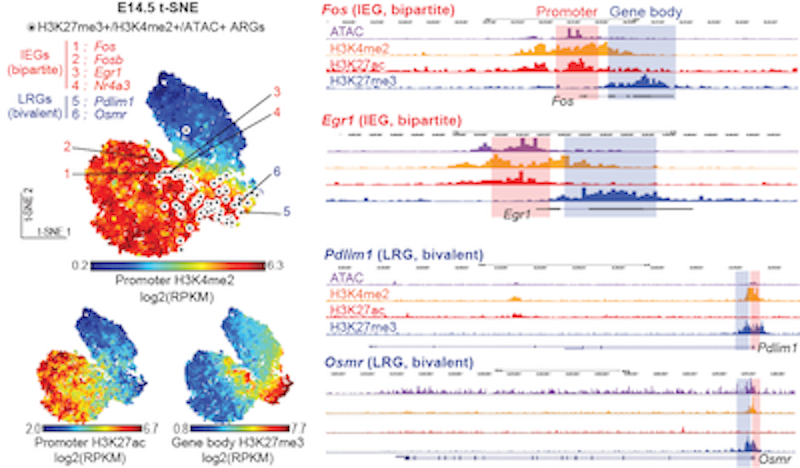
The number and type of genes can change during a time course. For the first few minutes up to one hour, there is a certain type of genes called immediate early genes or IEGs expressed. I studied these in my postdoc research. There are 10 to 20, not hundreds, of IEGs. These are transcription factors that regulate hundreds of downstream genes.
In this way, just a small number of genes change at first, and then gradually more changes take place within hours. It’s still not clear how many genes will be differentially expressed in the longer term, on the order of days.
Interestingly, not all neurons change in the same way. There is evidence that different types of neurons respond differently, transcriptionally, to environmental stimuli. Dr. Kitazawa explains why this is fascinating to the contemporary neurobiologist:
Single cell sequencing technologies are developing very fast. Analyzing gene changes at the single cell level allows us to discriminate between how neurons react. It's clear that all neurons do not necessarily act in the same manner. We used to say that the IEGs might be similar, but it's becoming clear that that is not even correct.
Molecular profiling for memory formation
Single cell genomics has indeed evolved rapidly. When Dr. Kitazawa started postdoctoral research in 2015 at Friedrich Miescher Institute for Biomedical Research (FMI) in Switzerland, single cell sequencing of RNA was available, but single cell epigenomics, such as determination of the methylome or chromatin accessibility, was difficult. Nowadays, such analysis, e.g., by single cell ATACseq, is commercially available.
These technologies are essential for Dr. Kitazawa to profile neuroplasticity at the single cell level for epigenetic and transcriptional events in memory formation, work supported by his ERC Starting Grant, ‘MemoPlasticGenomics’. Focusing on the adult brain, the group utilizes existing state-of-the-art technologies to investigate how memories are stored in so-called engram cells. Dr. Kitazawa describes these specialized neurons:
Engram cells have emerged as the cellular substrate of memory. This concept is very old, more than 100 years old. But about 10-15 years ago, Susumu Tonegawa (Nobel Prize for Physiology or Medicine in 1987), and Sheena Josselyn really started to identify these cells as cellular substrates. Basically, engram cells are neurons that are transiently activated during a learning experience.
For example, if a mouse is exposed to an environmental cue, such as a sound, that is coupled to an electric foot shock, then the mouse will associate the cue with the adverse experience. The mouse learns that the environmental cue can cause pain. During such trials, certain neurons get activated, and the memory is stored. The next time it hears the sound, the mouse does not move - a fear response. The exact same neurons that are activated the first time tend to get reactivated upon memory recall.
“Using optogenetics to selectively and artificially activate those neurons resulted in the memory being recalled. Just stimulating the cells can make the mice anxious or threatened. So, just by manipulating the activity of these neurons, we can see the artificial expression of a memory,” explains Dr. Kitazawa.
And, if the engram cells die, this memory would be erased.
One of the main pillars of Dr. Kitazawa’s research program is to investigate the molecular profile, the single cell epigenome and transcriptome, of such engram cells.
To get there, he builds on successful epigenetic and transcriptional profiling of IEG expression in maturating somatosensory neurons, in which he discovered a novel mechanism regulating IEG inducibility, work published last year in Nature Genetics. He explains:
IEGs are used to define and label engram cells, sort of like an expression signature. In my postdoc work at FMI, I discovered a novel and peculiar epigenetic signature, a combination of different histone modifications in a promoter and gene body. I named it ‘bipartite chromatin-regulating IEGs’.
He’s also interested in the development of new genomics approaches that can overcome the limitations of existing technologies, so that he can address the difficult questions that elude researchers today.
A multidisciplinary team at DANDRITE
Considering the interdisciplinarity of Dr. Kitazawa’s goals and approaches, his research spans from neurobiology to epigenetics to genomics bioinformatics, and he needs experts in a range of areas from mouse behavior and surgery to electrophysiology and optogenetics to single cell genomics and bioinformatics. Luckily, expertise in many of these areas surrounds Dr. Kitazawa - at DANDRITE and in the local research community in Aarhus.
At DANDRITE, and the PROMEMO offshoot, there are neurobiology experts, especially with a focus on memory from a variety of aspects - Poul Nissen in structural biology, Sadegh Nabavi with circuit biology, Tomonori Takeuchi, and Marco Capogna in memory engram cells, and Anders Nykjær with receptors required for neuroplasticity.
DANDRITE’s Scientific Advisory Board (SAB) also caught Dr. Kitazawa’s interest. For example, Cornelius Gross, a group leader at EMBL-Rome and DANDRITE SAB member has already stimulated Dr. Kitazawa’s research with input during a recent SAB visit.
Rounding out his team will be expertise in bioinformatics, beginning with a new PhD student in the spring. He will likely expand this area of his group. And, he sees collaboration potential both in the Nordic EMBL Partnership and with researchers at EMBL sites: “I'm very much interested in the genomic biology unit at EMBL-Heidelberg. So far, I don't have a clear idea for collaboration, but it’s good to have such strong science in partnership with DANDRITE.”
From morphology to neurobiology
Some scientists are drawn into a research field early and spend their entire career in that arena. Others tend to follow the science to where it takes them, even meandering among fields. Having started his education as a student of law, Dr. Kitazawa only found science in his early 20s. When he did, there was no looking back. He went to medical school, in the MD-PhD track and was lured into research. He explains how he became a neurobiologist:
I was interested in the brain from the beginning. But when I was a medical student, I needed to study human anatomy, dissecting bodies, and I got interested in morphology. At that time I decided to go to mammary and craniofacial morphogenesis for the PhD. But I was still thinking about neurobiology. My postdoc supervisor, Prof. Filippo M. Rijli at FMI studied both morphogenesis and neurobiology. It was an ideal place for me. I eventually launched research in neuronal circuit development.
Old interests in morphogenesis and developmental biology still linger, and Dr. Kitazawa even found himself publishing a first author paper in the field still this year.
The burden of memory loss and the pain of not being able to forget
While his research is fundamental - finding the molecular paths to neuroplasticity, Dr. Kitazawa is well aware that discoveries from his work could eventually make a difference for society:
Studying memory and plasticity helps us to understand certain types of dementia. For example, in an Alzheimer’s disease mouse model, it was reported that engram cells have some deficiency. The cells can still stock memories, but somehow environmental stimuli cannot activate the cells to recall a memory. So the connection from environmental cue or experience to engram cells is disturbed.
The cells are still there, but the connection to them is broken. Memory deficit is clearly related to neuroplasticity, and dementia is becoming a big problem of an aging society, for both quality of life and for the economy. Dr. Kitazawa expounds on the impact:
As the population ages and we live longer, there needs to be more and more facilities to be able to support people with advanced dementia. It's a very big problem in Japan. Pension funding is not working anymore. I think it's working well in Nordic countries. But, we don’t know how it will go. Science technologies can help to sustain our quality of life in society.
On the flip side, there can be pain from not being able to forget. For example, people fighting post traumatic stress disorder can’t forget a traumatic memory. So, being able to avoid unnecessarily memory recall is also important.
Regardless of the need to remember or to forget, the fundamentals of long-term memory formation are sure to be clarified in the years to come.
Brief career summary
Dr. Taro Kitazawa was initially trained in medicine and biosciences at the University of Tokyo. In 2014, he completed a Ph.D. degree in evolutionary biology, including the developmental analysis of mammary and craniofacial morphogenesis with Prof. Hiroki Kurihara at the Graduate School of Medicine, University of Tokyo. He began postdoctoral work at the University of Tokyo in the same year. In 2015, having been awarded a Japan Society for the Promotion of Science (JSPS) fellowship, he relocated to the Friedrich Miescher Institute for Biomedical Research (FMI) in Switzerland to continue postdoctoral research with Filippo M. Rijli. His postdoctoral work examined the epigenetic and transcriptional regulation of neuronal activity-responsive genes during the maturation of sensory neurons. Since summer 2022, Dr. Kitazawa has been a DANDRITE Group Leader and Associate Professor at Department of Molecular Biology and Genetics at Aarhus University in Denmark. His work is supported by a prestigious ERC Starting Grant entitled ‘MemoPlasticGenomics.’
The article is published as part of the “Behind the Science'' profile series, taking an in-depth look at a scientist or group within the Nordic EMBL Partnership.