
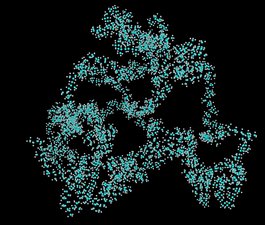
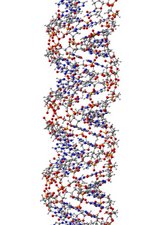
Polymers are substances composed of macromolecules, very large molecules with molecular weights ranging from a few thousand to as high as millions of grams/mole. It is composed of many repeated subunits. The IUPAC definition of a macromolecule is: “A molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass.” Natural polymers include protein, starch, cellulose, DNA and make up most of the structures of living tissue. Because of their broad range of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life.
Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass relative to small molecule compounds produces unique physical properties. The first, wholly synthetic polymer material, phenol-formaldehyde resin (Bakelite), was developed from 1905 to 1909 by Baekeland. The initial developments of synthetic poly-condensation and poly-addition polymers occurred from about 1928 to 1947 following a seminal paper by Staudinger in 1920.Synthetic polymers now constitute one of the most successful and useful classes of materials and possess a broad range of physical properties - including cationic properties resulting on cationic polymers.
For more information about polymers please visit: https://iupac.org/polymer-edu/what-are-polymers/.