Participants: Per Nørnberg and Haraldur P. Gunnlaugsson
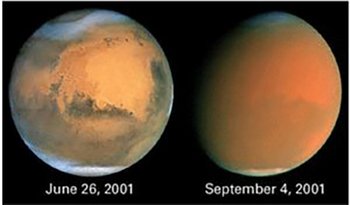
Mars is covered by fine red dust. This dust gives the planet its characteristic color. The dust is present in the atmosphere, dominating the weather and sometimes becoming so thick that it plunges the planet into darkness. Its origin, its physical, chemical and geological properties have all yet to be fully understood.
Central to dust research and instrument tests the Mars group has developed two unique recirculating wind tunnels housed in environmental chambers. Here parameters such as: gas pressure, gas composition, temperature and wind conditions can be controlled to reproduce those found on Mars. Importantly, special analogue dust material can be injected to simulate the Martian atmospheric aerosol. Experiments can be set up or Mars lander flight instruments can be exposed to the dusty conditions like those found on the Martian surface. Physical properties of analogue dust can be studied with a variety of techniques. The responsibility for running the wind tunnel laboratory under Institute for Physics and Astronomy is in the hands of Dr. Jonathan Merrison.
Participants: Per Nørnberg and Haraldur P. Gunnlaugsson
The red planet has its name from the reddish colour by which Mars appears in the sky on clear evenings. The reason for this colour is a layer of surface dust on Mars consisting of iron oxides, and it was determined by Earth-based reflection spectroscopy and confirmed by optical studies on the Viking-missions and the Pathfinder mission in 1997 from which the photo below is taken (Pathfinder view).
A number of iron oxides are known from Earth to be ochreous or reddish/yellowish coloured (Hematite, Maghemite, Ferrihydrite, Goethite and Lepidocrocite). Common to the reddish or yellowish iron oxides are that they contain iron in the oxidation state +III. The presence of iron oxides on the surface of Mars is no surprise since iron (Fe) is known to be the third most common element (after oxygen (O) and silicon (Si)) of the planet’s surface material.

However, the primary minerals just below the visible surface of Mars are known to consist almost entirely of iron in the oxidation state +II. This is known partly from studies of Martian meteorites, which are assumed surface rocks ejected from Mars upon meteorite impacts at the planet, and partly from studies of reflection spectra taken of the Martian surface. Thus, during formation of the Martian surface dust, oxidation of the iron from +II to +III took place. The red colour is a much bigger challenge after the NASA Mars Exploration Rovers determined the chemistry of the dust captured from the atmosphere to be nearly the same as the basic rock.
Basically, two possibilities are discussed:
The dust particles at the surface of Mars, in their mineralogy, hold information on their formation history, and thus information on the earlier surface environmental conditions. However, today’s knowledge of the Martian dust operates with particles about 2 microns in size, with a complicated basaltic mineralogy. In an Earth environment, such small size particles would disappear within decades or a few hundred years by weathering. This is an important reason why missions to Mars study the dust in details.
As samples of the red dust from Mars are not available, background studies and tests of experiments to be carried out on Mars missions have to take place on Earth by using Mars analogue samples.
None has all the properties of the Martian dust of these samples. One dust analogue is found in Denmark (Salten Skov l). First of all, it has the right grain size distribution (about 2-3 µm particles) and magnetic properties (3.9 Am2/kg) not too far from the Mars dust (~2 Am2/kg). The near UV spectrum of the dust is comparable to spectra from the surface material of Mars. Electrification, adhesion and cohesion properties are as we expect the Mars dust to have under the cold and dry planetary conditions. However, the mineralogy is goethite (~75%), Hematite (~19%), Maghemite (~6%) and thus far from the Martian dust with a basaltic chemistry.


Mars sample analogues have been used in scientific experiments related to Martian surface processes (magnetic capture, electrification, adhesion, aerodynamics, Mars mission instrument and solar panel tests). The main facility used for dust experiments are two Mars wind tunnels (Jonathan P. Merrison).
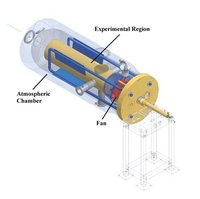
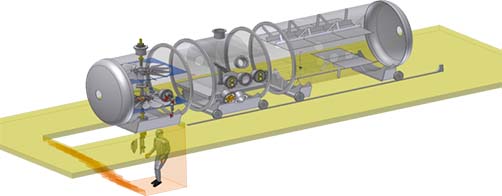
However, a number of other experimental instruments are already described, and analytical instruments necessary for characterization of the mineralogical properties are at our disposal. Particle size of the dust is determined by laser diffraction. Mineralogy is determined by x-ray diffraction (XRD) and Mössbauer spectroscopy (MS), and morphology and chemical composition by scanning-(SEM) and transmission electron microscopy (TEM), both with EDX analyzers (energy dispersive x-ray analysis), and total chemical analyses of bulk samples by x-ray fluorescence (XRF). UV-Vis spectrometry, NMR spectroscopy and Raman spectroscopy are also methods available to the group. Most recently in studying of sediment surface transformation in saltation processes where mineral surfaces are triboelectrically charged, which transforms both solid phases and surrounding gasses.
Iron oxides
References:
[1] Nørnberg, P. et al. (2009), Planet and Sp. Sci. 57, 628-631.
[2] Nørnberg, P. et al. (2009), Clay Minerals. 44, 239-247.