The question of the origin of life is one of the most fundamental questions in science. Of all other planets in our solar system, Mars is the most Earth-like and remains our best chance to find life, presumably microbial life, which, on Earth, is found in even the most extreme environments.
The study of Mars requires the full spectrum of investigative techniques known to science. It is by nature cross-disciplinary, which is reflected in our research team including institutes of physics and astronomy, geology, chemistry and biology. Such collaboration is vital if we are to answer the questions of our origin and the possibilities of extra-terrestrial life.
In addition, in the aspect of the presently very intensive study of exoplanets, all indications of life under extreme conditions are scientific “must” studies.

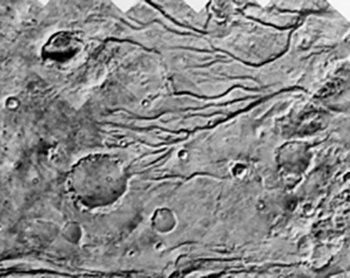
Vital to all forms of life on Earth is the presence of liquid water. The most recent orbital and ground based observations of Mars by the Martian satellites and rovers on the surface have made astonishing discoveries of vast ancient oceans, riverbeds, lakes and flow channels. In addition, huge quantities of subsurface water (ice) have been discovered spanning the entire range of Martian latitudes. A challenge for simulation experiments is now to find under which conditions, how and where liquid water can be found on Mars. Resent observations of short living streams has been observed on the surface of the planet. This indicate, that the water known to be present in form of ice close to the surface, now and then, maybe not that unusually, melts and appears on the surface.
The heavy bombardment period, by the end of the solar system formation, where the gravitation fields of the planets collected most of the material that had not until then ended up in planets, exposed the planet surfaces and atmospheres to very strong erosion. A big part of the Mars atmosphere was lost in that process.
Current models of early Mars by the end of the heavy bombardment (3.8 billion years ago) show it to be a much warmer and wetter planet than today. The models predict a higher atmospheric pressure at that time; temperatures above zero and oceans of liquid salt water. This resembles a hydrological cycle like the one known on Earth today and would cause a strong weathering environment. It could have been an environment capable of supporting life.
Loss of a protective magnetic field and reduction of volcanic outgassing left the atmosphere unprotected to UV radiation and the solar wind erosion of light gasses and molecules like water, ammonia and methane, but leaving e.g. oxygen, nitrogen and carbon dioxide.
However, the fact that the Mars’ rotation axis is perturbing much more than the rotation axis of the Earth have given rise to models for changes of the surface climate on Mars within few millions of years (a million years is a long period in a human context, but not in a geological). If big parts of the ice on the poles of Mars evaporate when the axis is oriented closer to the Sun, this leads to a higher atmospheric pressure, which results in clouds of water that might fall as snow on high ground. Then over years, glaciers will form with an erosion environment very different from the Mars we see at present. Possibly close to the hydrological environment we know from Earth.
| Atmospheric Profiles: | Earth | Mars |
| Pressure (bars) | 1.013 | 0.007 |
| Carbon Dioxide % | 0.03 | 95.3 |
| Nitrogen % | 77 | 2.7 |
| Argon % | 0.9 | 1.6 |
| Oxygen % | 21 | 0.13 |
| Carbon Monoxide % | - | 0.07 |
| Water % | - | 0.03 |
| Planet data: | Earth | Mars |
| Mass (kg) | 6.0 x 1024 | 6.4 x 1023 |
| Diameter (km) | 12756 | 6787 |
| Mean density (g/cm3) | 5.52 | 3.94 |
| Escape velocity (m/sec) | 11200 | 5000 |
| Average distance from Sun (AU) | 1 (150 mill. km) | 1.52 (228 mill. km) |
| Rotation period (hours) | 23.93 (day) | 24.62 (sol) |
| Revolution period (days) | 365.26 | 686.98 |
| Mean surface temperature (oC) | +15 | (+20) -63 (-140) |


The surface of Mars today is a harsh place. The thin atmosphere (around 8 mbar of, mostly, CO2) does not act quite as efficiently as the greenhouse we know from the Earth’s atmosphere. The heat flux from the sun heating the planet surface leaves as long-wave radiation freely into space. Only close to the surface at low latitudes the temperature around noon will exceed zero degrees Celsius.
Because of the thin atmosphere, hard UV-radiation reaches all the way to the surface, and here the UV-radiation contributes to formation of highly reactive oxidative species. In this environment, organic matter will not survive UV radiation. Moreover, the thin atmosphere and lack of a global dipole field will allow Cosmic Rays and high energy solar radiation (primarily protons) to penetrate and interacts with atoms in the top surface producing strongly ionizing secondary radiation. In this radiation environment, it is difficult for the molecules necessary for biology, as we know it, to survive.
NASA’s Viking missions (1976), detected only trace amounts of organic matter on the Martian surface. Chlorinated methane compounds were detected, which led to speculations that it was left overs of solvents that had been used to clean the experimental apparatus before launch. Furthermore, the results indicated that the surface minerals were not only highly oxidized, but also highly oxidizing. Organic matter getting into contact with this surface material will release CO2 if heated. Since these experiments were performed on Mars there has been much debate about the cause of these oxidizing properties. But with the Phoenix mission an important clue was obtained. Perchlorate was found in concentrations of 0.5 – 1 % in the surface material. This finding has been corroborated by Curiosity’s analysis of soils in Gale crater close to the equator.
Looking at Mars with a pair of binoculars or a telescope reveals a reddish planet. Fine red dust covers nearly all the planet's surface and is dispersed in the lower atmosphere. From time to time storms entrain dust high into the atmosphere leaving the planet surface invisible for months. From the NASA Mars Exploration Rovers the chemical composition of the dust is known. However, the exact processes responsible for formation of the reddish colour coming from iron in oxidation state three is still unknown.
Laboratory experiments, spectroscopy, modelling, wind tunnel and environmental chamber experiments are all tools used by the Mars Simulation Laboratory, Aarhus University in projects to try to solve some of the challenging and important questions about surface processes on Mars.