Searching for grapevine fungal trunk pathogens on cover crop roots
The potential role of cover crops as alternative hosts for soil-borne fungi plant diseases has not been thoroughly explored. Root samples from cover crops from experimental plots in the CORE Organic Cofund BIOVINE project has been analysed to find out more.
It is well known that different soil-borne plant pests can be controlled by using diverse cover crops species in the inter-row space. For instance, Brassica spp. have been already investigated for their capacity to effectively suppresses soil-borne causal agents of Black-foot disease in vineyard soils. But the potential role of cover crops as alternative hosts for soil-borne fungi has been little explored. This information can be useful for a better understanding of groundcover management as a tool to manipulate soil-borne fungi in organic viticulture.
In the BIOVINE project (www.biovine.eu) analyses to determine the presence of Cylindrocarpon-like asexual morphs and also of Cadophora spp., Phaeoacremonium spp., and Phaeomoniella chlamydospora (causal agents of Petri disease of grapevines) on the roots of cover crops were set up. These fungi are known to live on dead organic material in soil to occur on dead plants, or act as weak pathogens of plants infecting wounds of roots and stems of various hosts through wounds and/or openings, thus being a potential source of inoculum for grapevine infections.
Research groups participating in the BIOVINE project have established experimental plots in France, Italy, Romania, Slovenia, Spain and Switzerland, including new viticultural systems based on increased plant diversity by planting selected cover crop species with the main goal to control arthropods, soil-borne pests (oomycetes, fungi, nematodes), and foliar pathogens. The Universitat Politècnica de Valencia (UPV) (Spain) has overseen the analysis of selected cover crops root samples grown in these experimental plots.
| FACT BOX: Method |
|---|
Cover crop plants were carefully dug out from the soil to keep the root system intact, introduced in plastic bags and stored in a fridge at 4 ºC until the moment to be processed at the UPV. In total, 44 cover crops samples were evaluated in 2019 for the evaluation of the presence of grapevine root and wood fungi on cover crop plants. These samples were collected in the experimental plots stablished in Italy (n=22), Romania (n=12), Slovenia (n=4) and Switzerland (n=6). Each sample corresponded to a different cover crop species and had 5 plants (220 plants in total). Fungal isolations were performed on Malt Extract Agar + Streptomycin Sulphate (MEAS). Plants were disinfected externally using bleach and washing twice with sterile distilled water. Fourteen roots fragments and seven internal crown fragments were plated per plant, incubated in darkness at 25ºC for 10-15 days, and fungal colonies transferred to Potato-Dextrose Agar (PDA) for sporulation and identification. For fungal identification, microscope observation of fungal colonies was performed aiming to a tentative grapevine trunk pathogens selection. Then, single spore cultures were obtained from selected colonies, which identity was confirmed by using gene sequencing (ITS and HIS-3 regions were studied). |
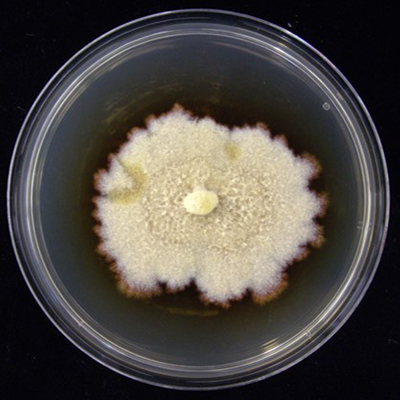
From the samples, 12 Cylindrocarpon-like asexual morphs isolates were obtained, all belonging to the species Dactylonectria torresensis (Figure 1). This species is a pathogen associated with black-foot disease of grapevines worldwide. In fact, among the causal agents of this disease, D. torresensis has been reported as the most frequent species. Moreover, D. torresensis showed some preference to colonize white clover (Trifolium repens), because 5 out of 12 isolates were obtained from this cover crop species from samples collected in Italy, Switzerland and Romania. Any other grapevine trunk were not detected in any of the cover crop samples.
Although the isolation of trunk pathogens was very low, these results showed that some cover crop species can act as alternative hosts for the species D. torresensis. Additional surveys will be conducted during the 2020 growing season in order to collect additional samples and perform new analyses.
Relevant links
BIOVINE project website, www.biovine.eu
Agustí-Brisach C. and Armengol J. 2013. Black-foot disease of grapevine: an update on taxonomy, epidemiology and management strategies. Phytopathologia Mediterranea, 52: 245-261.
Berlanas C., Andrés-Sodupe M., López-Manzanares B., Maldonado-González M.M. and Gramaje D. 2018. Effect of white mustard cover crop residue, soil chemical fumigation and Trichoderma spp. root treatment on black-foot disease control in grapevine. Pest Management Science, 74: 2864-2873. doi: 10.1002/ps.5078
Gramaje D., Urbez-Torres J.R. and Sosnowski M.R., 2018. Managing grapevine trunk diseases with respect to etiology and epidemiology: current strategies and future prospects. Plant Disease, 102: 12–39. doi: 10.1094/P DIS-04-17-0512-FE
Vukicevich E., D. Lowery T., Bennett J.A. and Hart M., 2019. Influence of groundcover vegetation, soil physicochemical properties, and irrigation practices on soil fungi in semi-arid vineyards. Frontiers in Ecology and Evolution. doi: 10.3389/fevo.2019.00118
Authors info
Josep Armengol, Instituto Agroforestal Mediterráneo, Universitat Politècnica de València, jarmengo@eaf.upv.es, http://iam.webs.upv.es
Antonio Ramón-Albalat, Instituto Agroforestal Mediterráneo, Universitat Politècnica de València, anraal@upvnet.upv.es,
Paloma Abad-Campos, Instituto Agroforestal Mediterráneo, Universitat Politècnica de València, pabadcam@eaf.upv.es, http://iam.webs.upv.es
Maela León, Instituto Agroforestal Mediterráneo, Universitat Politècnica de València, mleon@uv.es, http://iam.webs.upv.es
Mónica Berbegal, Instituto Agroforestal Mediterráneo, Universitat Politècnica de València, mobermar@etsia.upv.es, http://iam.webs.upv.es
Editor: Karin Ullven / Design: Christine Dilling
